
About us
Dianosic designs transformative intranasal solutions changing physicians experience and supporting a rapid return of patients to daily life activities.


2024
- Dianosic poursuit son plan préclinique comme prévu et confirme ses bons résultats initiaux pour le programme DIA01-R.
- Un nombre croissant de centres cliniques participent à l’étude observationnelle CAVI-T.
- Les premières études de formulation dans les domaines thérapeutiques du système nerveux central (SNC) (schizophrénie, Parkinson) ont été initiées.
2023
- Dianosic lève 4,7 millions d’euros pour préparer un essai clinique sur la rhinite chronique.
- Lancement du nouveau ballon CAVI-T doté d’une fonction anti-migration.
- Résultats précliniques in vivo prometteurs pour le DIA01-R.
- Dianosic ajoute le système nerveux central comme nouveau secteur d’activité et renforce sa plateforme DIAXX.
2022
- Dianosic figure parmi les 10 meilleures startups européennes du secteur des technologies médicales.
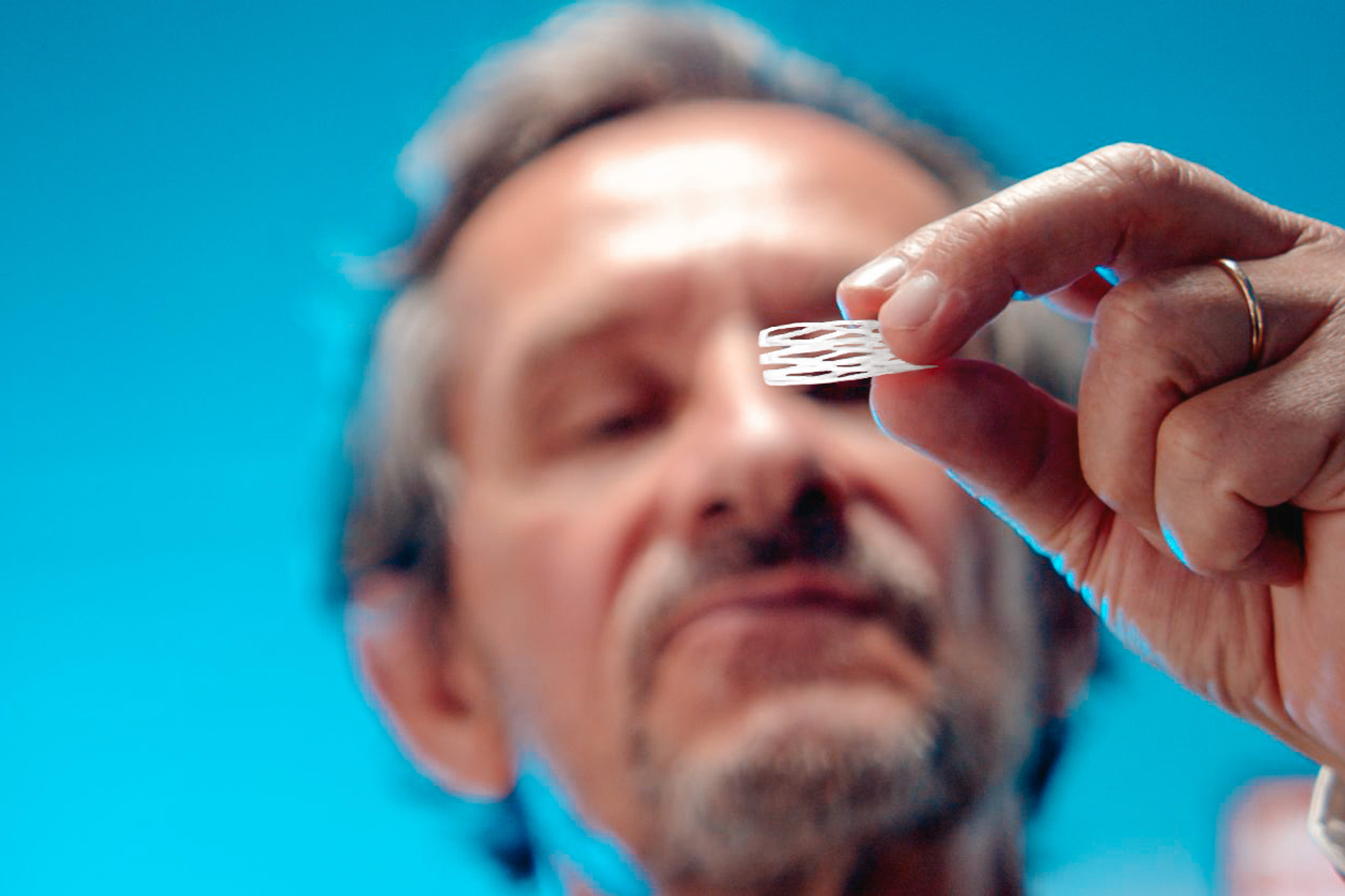
- Conception d’un prototype entièrement fonctionnel de la solution DIAXX pour la rhinite allergique chronique (DIA01-R)

2021
- Dianosic reçoit le prix « favori du public » du SNITEM.
- Lancement réussi de CAVI-T en Europe après la pandémie de COVID.
- Lancement d’outils de formation numérique pour CAVI-T.

2020
- Dianosic clôture un premier tour de financement de 1,5 million d’euros.
- Publication des premiers résultats cliniques pour CAVI-T.
- Marquage CE de CAVI-T et certification ISO 13485.

2019
- Dianosic remporte le prestigieux concours d’innovation I-Lab 2019.

2018
- Dianosic déménage à Strasbourg.

2017
- Développement du ballonnet intranasal asymétrique CAVI-T, une solution minimalement invasive pour améliorer le traitement des épistaxis.
- Le programme de développement de l’échafaudage intranasal résorbable actif (DIAXX) débute dans le traitement de la rhinite et de la sinusite.

Origine
- Marc Augustin (Président), Philippe Bastide (PDG) et le Pr Christian Debry (chirurgien ORL) créent Dianosic à Paris pour répondre aux besoins non satisfaits en otorhinolaryngologie (ORL) / maladies respiratoires, notamment la rhinite allergique chronique, la rhinosinusite chronique et l’épistaxis.
Dianosic's Commitment to Enhancing respiratory care
Les affections ORL (oto-rhino-laryngologie) sont la principale cause de consultation médicale. Des améliorations significatives sont possibles pour répondre aux besoins cliniques non satisfaits. Dianosic collabore avec les professionnels de santé afin de proposer des solutions ORL de pointe qui transformeront la vie des patients souffrant de rhinite chronique, de rhinosinusite chronique et d’épistaxis. L’entreprise s’engage à :

Optimiser la qualité de vie des patients, en favorisant un retour rapide aux activités quotidiennes.

Optimiser l’efficacité et la sécurité grâce à une administration ciblée de médicaments à faible dose.

Tirez parti de notre plateforme d’administration intranasale de médicaments pour traiter les maladies du système nerveux central.

Réduire la durée et la fréquence des hospitalisations.

Discover our programs: DIA01-R, DIA02-S and more
Dianosic développe une plateforme d’implants intranasaux résorbables actifs (DIAXX) visant à administrer une dose réduite et ciblée de médicament dans la cavité nasale grâce à une solution simple et rapide à insérer. DIAXX offre un traitement à libération prolongée, révolutionnant la prise en charge des maladies chroniques telles que la rhinite et la rhinosinusite. La technologie innovante de la plateforme DIAXX est hautement adaptable et prometteuse dans le domaine du système nerveux central, exploitant le concept « du nez au cerveau » dans la schizophrénie, la maladie de Parkinson, etc.
Qualified team with complementary profiles

- Seasoned entrepreneur
-
>20 years of experience in Finance and International Development (Stellantis, IBM)
-
Member of the executive committee of the health insurancel company UNEO
-
Merger Acquisition / fundraising advisor (ACM partners)
- Linkedin account: https://bit.ly/3EvE9u1

-
> 20 years of experience in Medtech and Pharma (Pfizer, Johnson & Johnson, Livanova)
-
Business Unit Lead at Acclarent (ENT Division of Johnson & Johnson)
- Expertise in Market Access (pricing & reimbursement), Business Development and Professional Education
- Linkedin account: https://bit.ly/3sRamJv

- 10 years' experience in medical devices and pharmaceuticals (Roche)
- Expertise in quality management & regulatory affairs
- Doctorate in Biology from the University of Strasbourg (CIFRE SANOFI)
- Linkedin account: https://bit.ly/44RT6l2

-
14 years of experience in pharmaceutical development, generics, orphan drugs and combination products
- CMC Regulatory Project Management invariuous companies (INNOTHERA, NOVARTIS, OT4B )
- Expertise in product development and analytical chemistry
- Linkedin account: https://bit.ly/44GfrSh

-
Quality Manager, Clinical Operations Engineer
- 10 years' experience in research projects (University of Strasbourg)
- Expertise in in vivo studies
- PhD in Pharmacology + Diploma in Clinical Research
- Linkedin account: https://bit.ly/3sQRomk

- Healthcare engineer (Master's degree in healthcare engineering, medical devices option, Montpellier, DEDIENNE SANTÉ)
- Expertise in medical device (or healthcare product) design
- Specialized in biodegradable polymers and active ingredient release
- Linkedin account: https://bit.ly/3LfIgOD

- Over 20 years' career in the healthcare industry
- Solid experience in general management, operational strategy, business development, marketing and sales management at international level, across regions (Johnson & Johnson).
- Linkedin account: https://bit.ly/3EvE9u1

- Student at ESSCA business school, Master international Business
- Professional experience in the Medtech world (Johnson & Johnson)
- Linkedin account: https://bit.ly/3ZgllbS
- >20 years experience as chartered accountant, statutory auditor and Financial lead in Medium Caps and startups (Cléry, CTG Group, TheUpScalers).
-
Linkedin account:
https://www.linkedin.com/in/christophe-kica-50705218/

-
ENT Surgeon, University Hospital of Strasbourg, France
- Rhinology and skull base surgery expert
- Co-Director of the INSERM 1121 Research Unit (“Bioengineering and biomaterial)
-
Co-Director of the lab for skull base surgery (IRCAD)
- Board member of the French ENT Society, French College of ENT and Head & Neck Surgery and the French Association for Pediatric ENT
- Founder of Protip Médical (World first implantation of an artificial larynx, 2012)
- Linkedin account: https://bit.ly/3P8tcn5

- ENT surgeon (USA)
- Founding Director of the Cleveland Nasal-Sinus & Sleep Center
-
Past President of the American Rhinologic Society
- Former Vice President of Medical Affairs at Acclarent (J&J)
-
Featured in the prestigious “Best Doctors in America” and “America’s Top Doctors” lists
- Investigator, lecturer and co-author of ENT reference articles

- ENT surgeon at Amsterdam University Medical Centres
- World renowned specialist in the field of sinus and skull base surgery
- Member of the key international Scientific Societies (ERS, EPOS, ARIA, GA2LEN)
- Author of more than 500 scientific articles

- Neurosurgeon, Lariboisière Hospital, APHP (Paris)
- Neurosurgeon, Lariboisière Hospital, APHP (Paris)
- President of the « Skull base » Committee of the Worldwide Federation of Neurosurgery (WFNS)
- Executive member of the European Skull Base Society (ESBS)

- Director of Endoscopic Skull Base Surgery (Stanford School of Medicine)
- Associate Professor of Otolaryngology – Head and Neck Surgery (Stanford School of Medicine)
- Expert in advanced endoscopic sinus and skull base surgery
- Member of the Board of Directors (American Rhinologic Society)
- Author of ENT reference articles

- Consultant ENT Surgeon at Guy’s and St Thomas’ Hospital (London)
- World renowned specialist focusing on rhinology and anterior skull base surgery
- Chair of the UK Rhinosinusitis Commissioning Guidance Group and the Treasurer for the European Rhinologic Society
- Claire has published over 150 papers in medical journals and 20 book chapters

- ENT surgeon (CHI Créteil, France)
- Specialized in rhinology and skull base surgery
- Reviewer in key ENT Journals (B-ENT, Am J Rhinol, Rhinology, Eur Ann of Otolaryngology, Intensive Care Medicine)
- Permanent member of the French Rhinology Association (AFR)
- Pr. Coste does not receive any financial compensation for his work as scientific advisor

- ENT surgeon (University hospital of Nantes, France)
- Expert in rhinology, head and neck surgery
- Member of Inserm Research Unit 1229 (Regenerative Medicine and Skeleton (RMeS))
- Member of the French Society of Rhinology and Head and Neck Oncology
- Pr. Malard does not receive any financial compensation for his work as scientific advisor

- Adjunct Associate Professor, Dept. of Otolaryngology, Emory University
- CEO, ENT of Georgia North
- General Secretary, Pan-American ORL Association
- Past Director, AAO-HNS Board of Directors
- International Steering Committee, AAO-HNS
- President of the 2022 Pan-American Congress of Otolaryngology
- Investigator and author of Otolaryngology reference articles

- HTA expert, Statistician and clinical trials methodologist (Paris, Fernand Widal, APHP)
- Former member of Marketing Authorization Commission and Medical Devices Commission (CNEDIMTS)
- Founder of the Medical Devices Evaluation Center (CEDM)
- Member of the Galien Awards jury
- Pr. Vicaut does not receive any financial compensation for his work as scientific advisor
-
> 20 years of experience in Medtech and Pharma (Pfizer, Johnson & Johnson, Livanova)
-
Business Unit Lead at Acclarent (ENT Division of Johnson & Johnson)
- Expertise in Market Access (pricing & reimbursement), Business Development and Professional Education
- Linkedin account: https://bit.ly/3sRamJv
- Seasoned entrepreneur
-
>20 years of experience in Finance and International Development (Stellantis, IBM)
-
Member of the executive committee of the health insurancel company UNEO
-
Merger Acquisition / fundraising advisor (ACM partners)
- Linkedin account: https://bit.ly/3EvE9u1
-
Alex Theodorakis is President, Aptar Pharma Prescription, he oversees global prescription pharmaceutical activities ($800M), including the Congers, Le Vaudreuil, Mumbai, and Suzhou sites. With over 25 years at Aptar, he began his career in technical support at BLM Associates (later Valois of America, now Aptar).
He subsequently held roles in sales and production across North America, worked in France at the Le Vaudreuil site, and later managed pharmaceutical and commercial operations in the United States before being appointed to his current position in 2017. -
Linkedin account:
https://www.linkedin.com/in/alex-theodorakis-b07b7837/
-
Jérémy Paulen is Director of Business Development for Allergy & White Spaces at Aptar Pharma. He specializes in innovation, strategic partnerships, and nasal drug-delivery technologies.
With over a decade of experience across pharma, medical devices, and consumer health, he focuses on translating advanced drug-delivery platforms into patient-centric and value-creating solutions. -
Linkedin account:
https://www.linkedin.com/in/jeremypaulen/
-
Jean-François Rax is Partner and Head of Seed Funding, specialized in life sciences. He joined Capital Grand Est in 2014 as Life Sciences Investment Director for the seed fund Cap Innov’Est and has served as a member of the Managing Board since 2020, overseeing the seed investment department (€87M under management).
Previously, he was a collaborator at Inserm Transfert Initiative, a French seed fund dedicated to life sciences and human health.
-
Linkedin account:
https://www.linkedin.com/in/jean-francois-rax-0946512/
-
Alexandre Koressios is an investment professional and venture capital executive. He is a Member of the Management Board at Finovam Gestion, a venture capital firm specialized in early-stage technology investments in French regions, where he leads the Grand Est office.
He began his career in audit at Deloitte before supporting entrepreneurs at Réseau Entreprendre Paris. He later joined Starquest Capital as Investment Director and participated in around thirty technology startup closings. Alexandre has invested in digital and healthcare companies and actively supports his portfolio through board participation and strategic guidance. -
Linkedin account:
https://www.linkedin.com/in/alexandrekoressios/
-
Séverine is Head of Global Asset Strategy for telitacicept at Vor Bio. Previously, she spent over 15 years at Bristol Myers Squibb (BMS), where she held several senior commercial roles globally in hematology, biomarkers, COVID-19 monoclonal antibodies, and Zeposia, and served as Chief of Staff to CEO Giovanni Caforio.
Her international experience has led her to leadership positions in France, China, and the United States, encompassing commercial excellence, portfolio strategy, and the management of international programs. She began her career at the Boston Consulting Group before holding senior commercial roles in the pharmaceutical industry (GSK, Sanofi, J&J). She is a graduate of HEC Paris and holds an MBA from INSEAD. -
Linkedin account:
https://www.linkedin.com/in/severine-lacourt-865763b/
-
Linkedin account:
https://www.linkedin.com/in/eric-brandt-6529613/
-
Clémentine Lombaert is an Analyst at Capital Grand Est, where she has been working since 2022 on seed-stage investments through the Cap Innov’Est fund.
She contributes to opportunity assessment, due diligence, and the strategic monitoring of portfolio companies. She holds a general engineering degree with a specialization in life sciences, completed following a final-year project in private equity.
-
Linkedin account:
https://www.linkedin.com/in/cl%C3%A9mentine-lombaert-/
We built a solid network of partners to support company funding and growth.
Nous sommes soutenus par des Business Angels, une plateforme de financement participatif de premier plan (Wiseed), la Fondation Force, BPI, Banque Populaire et Caisse d’Épargne Grand Est. Depuis sa création, le soutien de la Région Grand Est, de Quest for Health, de Biovalley France, de Conectus et d’Eurométropole a été d’une importance capitale.
Pour en savoir plus sur nos projets en cours, notre stratégie à long terme et nos partenaires, veuillez consulter notre page « Actualités et partenaires ». Rejoignez-nous et participez à l’aventure Dianosic !






