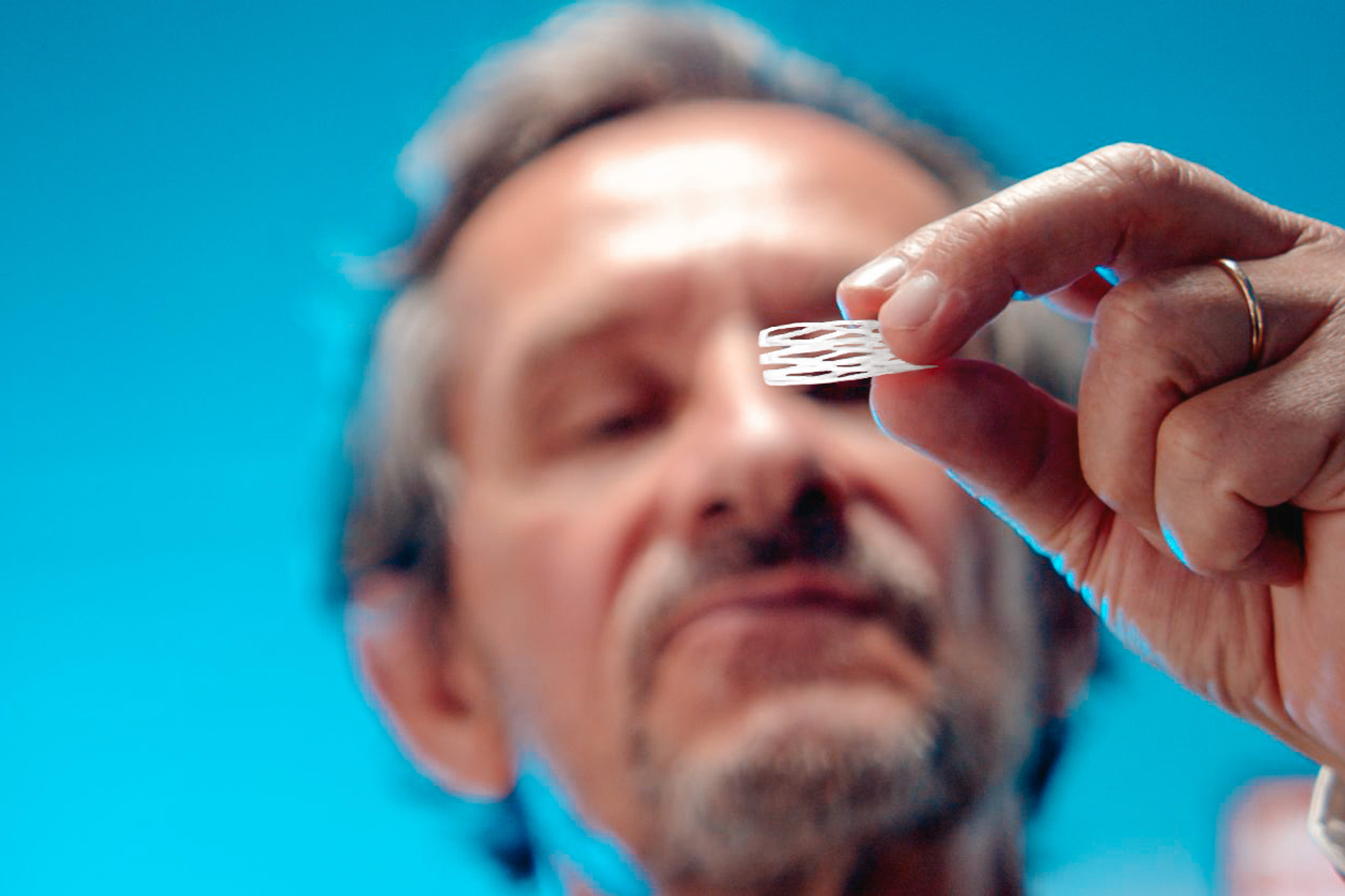
Our programs
Discover the future therapeutic solutions of chronic rhinitis and chronic rhinosinusitis - The Dianosic Active Resorbable Intranasal Scaffolds (ARIS).
Dianosic develops the innovative ARIS platforms that benefit from proprietary drug-eluting, long-term resorbable polymer.

There a is a need for treatment paradigm change to better manage patients, optimize treatment efficacy and safety.
85 % patients suffering from chronic rhinitis do not comply with their treatment.⁶
20 % patients suffering from chronic rhinosinusitis do not respond to intranasal corticosteroids.⁷
Only 20% of nasal spray active ingredients reach the targeted treatment area.⁸
Dianosic unique platform - Created to radically transform the management of these debilitating diseases
Delivering patient centric, “insert and forget” solutions maximizing patient quality of life and addressing physicians’ needs in terms of efficacy, safety, ease of use and patient adherence to treatment.

Long term, bioresorbable, unique product architecture, designed to optimally deliver a targeted, low dose of effective drug treatment in a controled and steady manner.
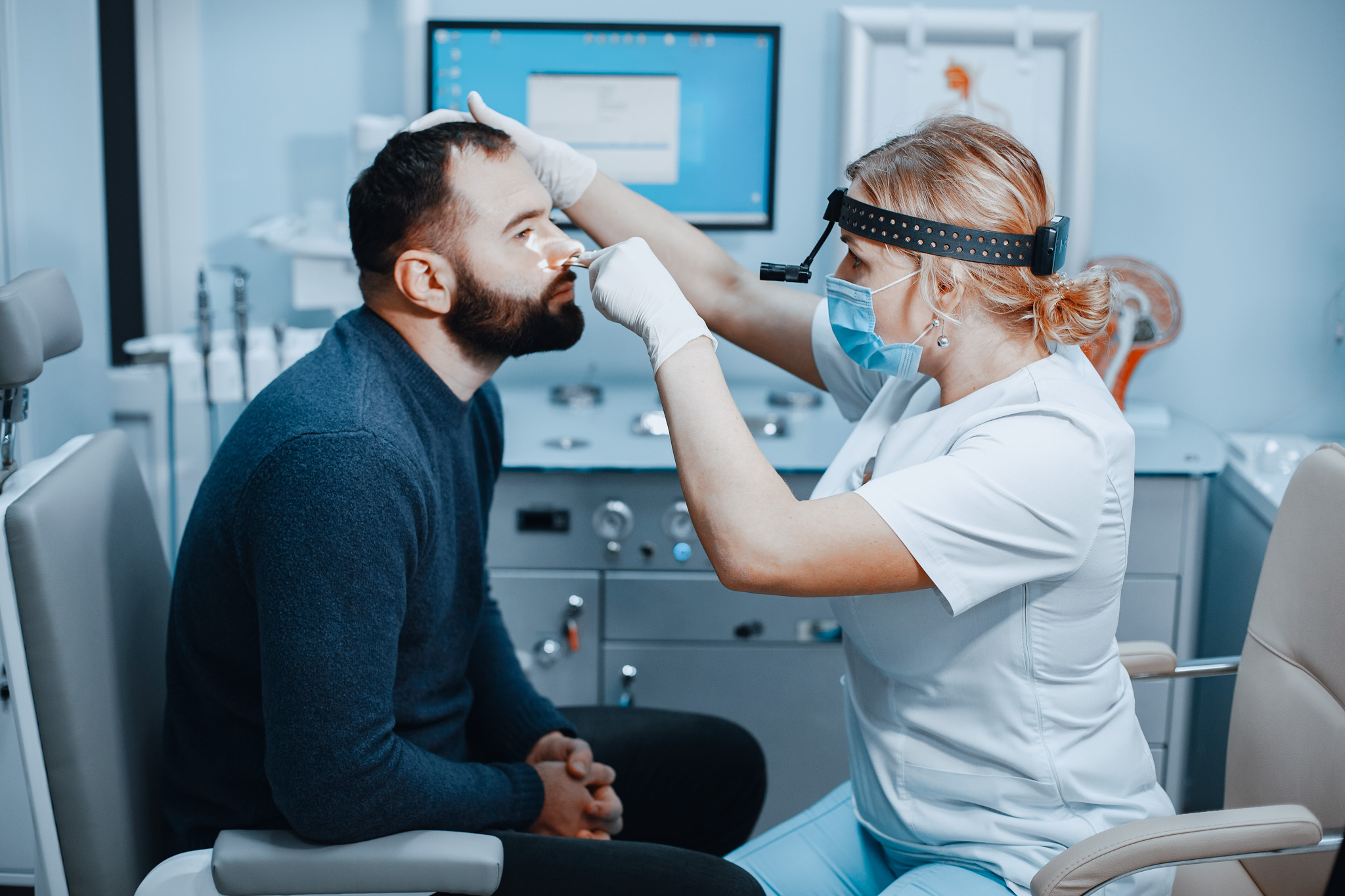
Implantable “in-office” under local anesthesia, generating significant savings for the healthcare systems.

100% treatment compliance (vs 15% for nasal sprays in rhinitis⁹) thank to our “insert and forget” solution.

Unique scaffold architecture combined with proprietary drug-eluting, long-term resorbable polymer
Our ARIS platform has been created to address both chronic rhinitis (ARIS-R) and chronic rhinositusitis (ARIS-S) and radically differentiate from existing solutions (resorbable scaffold, “insert and forget” approach, treatment duration, targeted, low-dose, sustained drug release) .
ARIS-R
Chronic rhinitis
ARIS-R is the ideal solution for controlling symptoms in persistent, moderate to severe allergic rhinitis. It delivers locally a low dose of corticosteroid for a duration of 6 months. ARIS-R offers an alternative to intranasal corticosteroids and to more invasive therapies and resolves patient adherence to medications challenges, thus avoiding symptoms aggravation.
ARIS-S
Chronic rhinosinusitis
ARIS-S is adapted to long-term symptomatic treatment of chronic rhinosinusitis in adults. It is placed into the nasal cavity under local anesthesia during a less than 30 minutes in-office procedure. ARIS-S delivers locally a low dose or corticosteroids for 12 months offering long-term symptoms relief for patients.
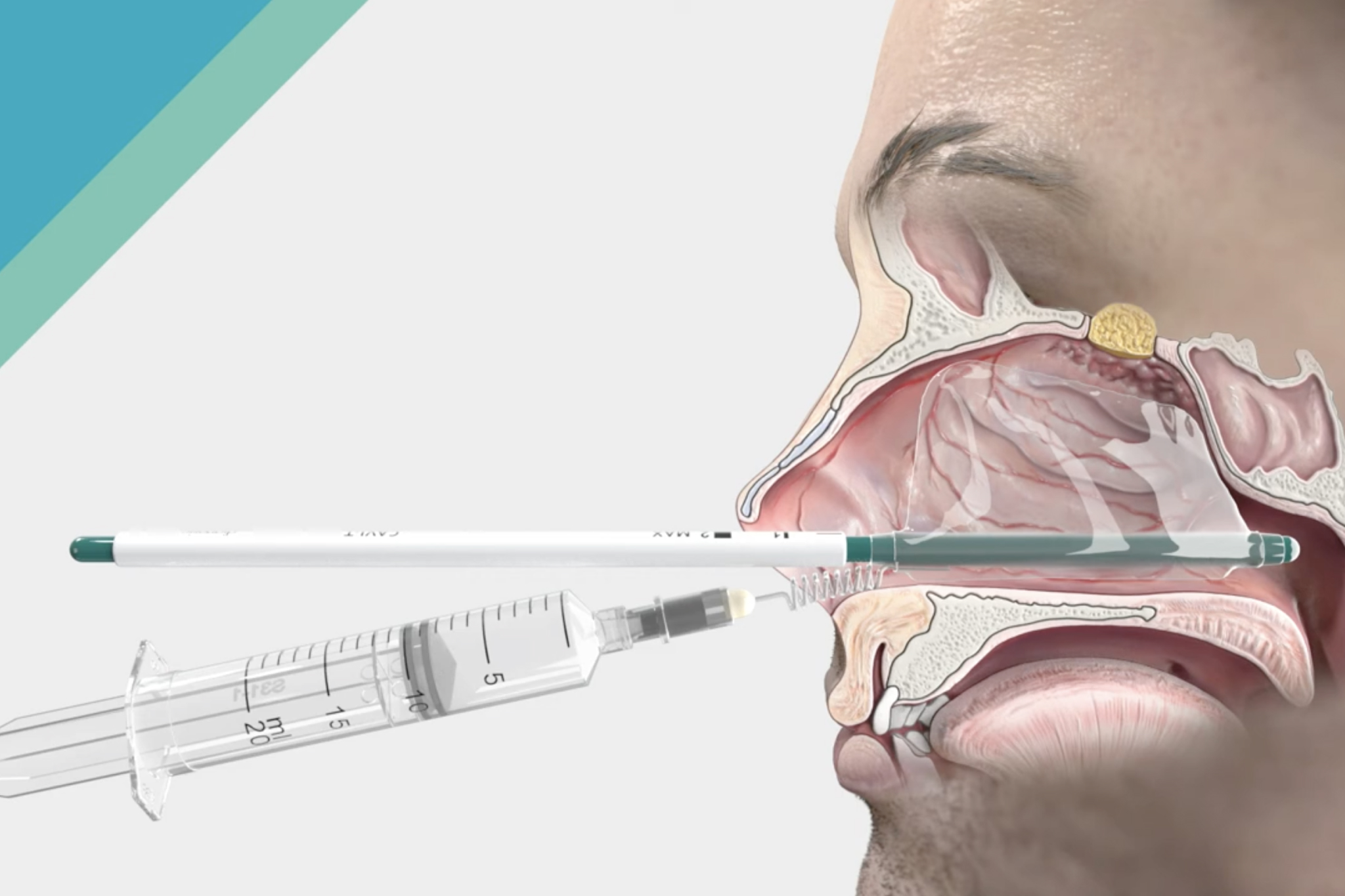
Discover our new solution for epistaxis – CAVI-T
Dianosic successfully developed and launched a versatile and easy to use solution designed to transform the management of intranasal bleeding (epistaxis). CAVI-T illustrates our continuous commitment to deliver innovative solutions to the ENT community.
³ Prevalence and Incidence of Diagnosed Chronic Rhinosinusitis in Alberta, Canada. Yuan Xu et al. JAMA Otolaryngol Head Neck Surg. 2016;142(11):1063-1069. doi:10.1001/jamaoto.2016.2227
⁴ Bauchau, V., & Durham, S. R. (2005). Epidemiological characterization of the intermittent and persistent types of allergic rhinitis. Allergy, 60(3), 350–353.doi:10.1111/j.1398-9995.2005.00751.x
⁵ Antonicelli L, Micucci C, Voltolini S, Feliziani V, Senna GE, Di Blasi P, et al. Allergic rhinitis and asthma comorbidity: ARIA classification of rhinitis does not correlate with the prevalence of asthma. Clin Exp Allergy. 2007 Jun;37(6):954-60.
⁶⁻⁹ Menditto E et al. Adherence to treatment in allergic rhinitis using mobile technology. The MASK Study. Clin Exp Allergy. 2019 Apr;49(4):442-460. doi: 10.1111/cea.13333.
⁷ The Role of Balloon Sinuplasty in the Treatment of Chronic Sinusitis. Cummings JP et al. JCOM. 2009; 16(1):30-6.
⁸ Computational Fluid Dynamics (CFD) has been used to investigate deposition patterns of particles from spray devices [12–19]. These studies found low deposition efficiencies in the middle to posterior nasal cavity region and sinuses, where absorption occurs.